April 2, 2012
Researchers Study and Develop Approach to Treat Mitochondrial Disorders
Share this story
Within each of our cells are a number of organelles governing operations – making sure we function as smoothly as possible. But one slip on the molecular level could mean disaster.
Researchers are now learning that when that slip occurs in the mitochondria – the organelle known as the “powerhouse” of the cell – a chaotic chain of events can unfold.
The mitochondria, which is a source of energy for the cell, is also responsible for cell signaling, differentiation and cell death. It contains its own set of blueprints in the form of mitochondrial DNA.
Evidence shows that a growing number of neurodegenerative, cardiovascular and metabolic-related diseases contain diseased mitochondrial DNA that cause mitochondria to malfunction.
And that’s where a multidisciplinary team of Virginia Commonwealth University researchers enters the picture.
The team, which includes researchers from the VCU Center for the Study of Biological Complexity, School of Engineering and the School of Medicine, has developed a therapeutic approach that may one day help treat neurodegenerative disorders that have defects in their mitochondrial genome and physiology. This may include disorders such as Leber’s hereditary optic neuropathy, which causes blindness in young adults, and Leigh’s syndrome, a fatal neurodegenerative disorder of infants.
Daily management of patients with mitochondrial and other degenerative disorders is a challenge. Although a number of approaches are being investigated, there is unfortunately no cure today for such diseases. Therefore the primary goal is of symptom control and improvement in the quality of life as much as possible for patients.
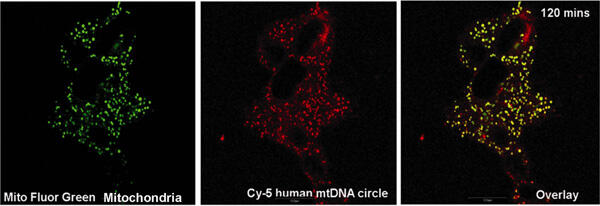
In a recent study, jointly led by Shilpa Iyer, research scientist at VCU Center for the Study of Biological Complexity, and James Bennett Jr., M.D., Ph.D., professor and chair of the Department of Neurology in the VCU School of Medicine, researchers employed mitochondrial gene therapy, which allows for introduction of external healthy mitochondrial DNA into mitochondria containing diseased mitochondrial DNA in living cells.
Iyer and colleagues reported that the healthy mitochondrial DNA in a complex with a recombinant protein, provided by Gencia Corp. in Charlottesville, succeeded in restoring a majority of the energy failure in cell lines generated from patients afflicted with Leigh’s syndrome or Leber’s hereditary optic neuropathy.
According to Iyer, the findings show that improving cellular respiration in this fashion may offer therapeutic options for patients suffering from these disorders.
“With this technology, the diseased mitochondrial DNA copies can be reduced and energy failure can be restored, paving the way towards treating mitochondrial DNA-based diseases,” said Iyer.
This study also demonstrated that mitochondrial biogenesis – the formation of new mitochondria - likely accounted for the increase in respiration.
“With the advent of this new technology, we will have a better understanding of the biology of mitochondrial function and be able to treat hereditary as well as sporadic mitochondrial and other degenerative diseases characterized by deficiencies of energy production,” she said.
According to Iyer, there is still more research to be done in this field and mitochondrial gene therapies in combination with other approaches may have the potential of being included in treatment strategies in the next five to ten years.
The study was published in the March issue of the journal Human Gene Therapy.
Other contributors to the study included Raj R. Rao, associate professor in the Department of Chemical and Life Science Engineering in the VCU School of Engineering; Erich Gnaiger, Ph.D., professor in the Department of Transplant Surgery at the Medical University of Innsbruck in Austria; and Gencia Corp. in Charlottesville, which provided the recombinant human protein used in the study.
The study was supported by grants from the National Institute of Health, the Parkinson’s Disease Foundation and the American Parkinson’s Disease Foundation.
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.










