May 18, 2022
From theory to practice: New stable, magnetic superatom could power innovations in nanomaterials
Share this story
Researchers from Virginia Commonwealth University, Columbia University and Harvard University have taken a superatomic principle, long explored by VCU researchers, from theory to practice by designing a stable, magnetic superatom — a discovery that could change how magnetic materials are made at the smallest level.
Superatoms are clusters of atoms that can act like elements on the periodic table, but with specific properties that are difficult to replicate using elements found in nature.
The structure of the superatom these researchers have developed can serve as a building block in the creation of new nanomaterials for data storage, cellphones, motors and sensors, said Shiv Khanna, Ph.D., Commonwealth Professor and chair of the Department of Physics in the VCU College of Humanities and Sciences. Khanna and his coauthors published their findings earlier this spring in the Journal of the American Chemical Society.
This extremely small, thermally stable superatom, which has a high magnetic strength, also has great potential for its use in semiconductors, components that are found in microchips, transistors and other devices and which, recently, have been hard to come by due to a global shortage. This superatom will be an innovation for which the uses are wide-ranging, Khanna said.
“As the pace of technology carries us toward smaller devices, there is a constant search for new magnetic materials,” Khanna said. “This can have potential applications in terms of switchable devices, electronics, catalysts for green energy and so on.”
Elements with filled subshells are more stable, as their electrons are distributed evenly, and in stable superatom clusters the principle is the same. However, most stable superatom clusters do not also have a large amount of magnetism.

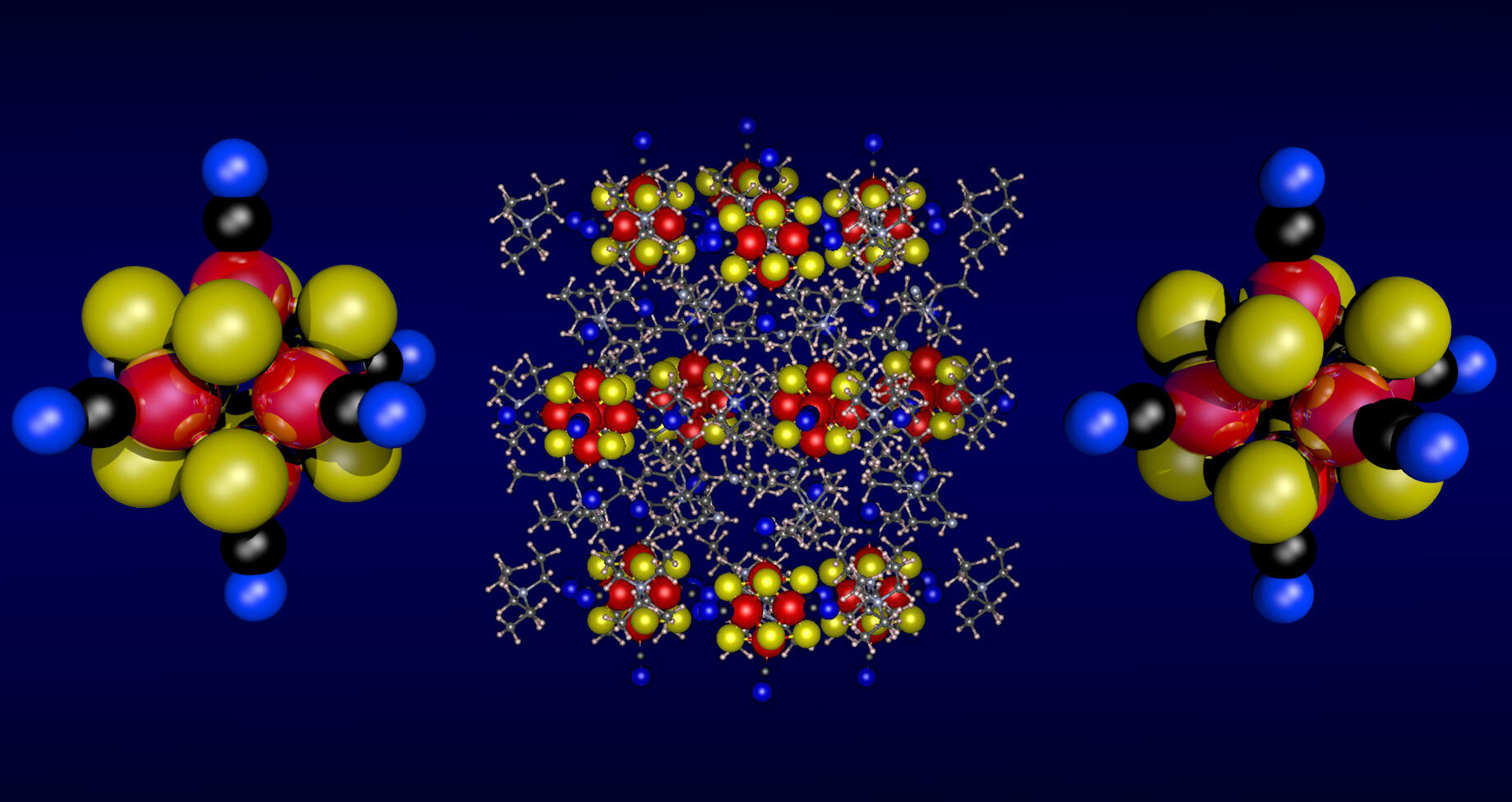
In their earlier work, Khanna and co-workers at VCU had predicted that a new type of metal chalcogenide superatom cluster could uniquely combine high stability and a large spin magnetic moment, a property that is responsible for the strength of an object’s magnetic field.
Based on this prediction, scientists at VCU, Columbia and Harvard created a unique superatom cluster with a filled subshell for greater electronic stability and a high-spin magnetic moment.
“You can make critical predictions but, unless somebody makes these things, a prediction is a prediction,” Khanna said. “So, our colleagues at Columbia actually made this thing. They did a lot of experiments and indeed verified everything we had predicted. That is why this paper became very important. This is the first time a cluster has become magnetic through this new phenomenon.”
In addition to its stable magnetic character, the new clusters can accept or donate electrons, which is vital for the transfer of energy that powers electronics, and they can be combined with other elements or superatoms while maintaining their internal structures. This superatom’s stability and magnetism can make it useful in creating materials with programmable electrical conductivity or catalysis. This superatom can also be used to make magnets at the molecular level.
The Journal of the American Chemical Society paper includes corresponding authors Khanna, Amymarie K. Bartholomew, Ph.D., and Xavier Roy, Ph.D., of Columbia’s Department of Chemistry; and co-authors Dinesh Bista, Ph.D., of VCU’s Department of Physics; Alexander P. Aydt, Ph.D., of Columbia; Kevin J. Anderton of Harvard’s Department of Chemistry and Chemical Biology; Daniel W. Paley, Ph.D., of Columbia; Theodore A. Betley, Ph.D., of Harvard; Arthur C. Reber, Ph.D., of VCU; and Vikas Chauhan, Ph.D., of VCU.
The development of the new superatom builds on VCU’s initial investigations of the theory behind superatoms, which began in the 1990s.
“This work started at VCU, so we are very proud. We build the theory, and then people take it on and do experiments,” Khanna said. “It’s gratifying, particularly when experiments verify what you predict. And then it becomes a joint effort, like it has with Columbia and Harvard, where they can actually make these superatoms. It’s remarkable.”
The editors of the Journal of the American Chemical Society selected the article to be featured in “Spotlight on Recent JACS Publication” (J. Am. Chem. Soc. 2022, 144, 5198-5199).
Subscribe to VCU News
Subscribe to VCU News at newsletter.vcu.edu and receive a selection of stories, videos, photos, news clips and event listings in your inbox.